Strength of Acids and Bases
Strength of Acids and Bases: Overview
This topic covers concepts, such as Universal Indicator, pH Scale, Strength of Acid and Base Solutions, pH of Neutral Solutions, pH of Acidic Solutions, pH of Basic Solutions, Variation of pH with Hydrogen Ion Concentration, Variation of pH with Hydroxide Ion Concentration, pH Range of Strong Acids, pH Range of Strong Bases, Importance of pH in Everyday Life, Importance of pH for Survival of Aquatic Life, Role of pH for Growth of Plants, Role of pH in Our Digestive System, Tooth Decay due to Change in pH & Role of pH for Self Defence of Animals and Plants etc.
Important Questions on Strength of Acids and Bases
A student added a drop of universal indicator to mL of given solution and found that a green colour is produced. The value of the solution will be
The value of pure water is
The ion concentration of a solution is . The pH value of the solution is .
Digestive fluids in the stomach have approximate pH of
The pH of the solution having hydrogen ion concentration will be:
Given of a solution is and it is mixed with another solution having . The resultant of the solution will be
Which of the following is the strong a base:
pH of 0.001 M NaOH will be
A milkman adds a very small amount of baking soda to fresh milk, why?
Tooth enamel contains
A solution turns red litmus blue, its is likely to be
Fresh milk has a of . When milk changes into curd. The value will
The nature of calcium phosphate present in tooth enamel is
A solution turns red litmus into blue, its pH is likely to be
In certain solutions,
(i) moles of H+= moles of OH-
(ii) pH < 7
(iii) pH = 7
(iv) moles of OH- > moles of H+
Which of the above conditions refers to a neutral environment in standard conditions?
Self-ionisation equation of pure water is as follows.
(i) Pure water is neutral.
(ii) The number of H3O+(hydronium) ions is equal to the number of OH-(hydroxide) ions.
(iii) Pure water ionises very little.
Which of these statements are correct?
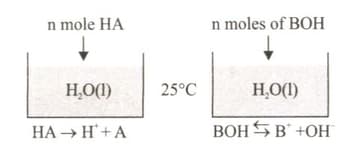
In Figure - 1 and Figure - 2, equal moles of and substances are added to the same volume of pure water at the same temperature. The equations for the solubility of these substances in water are given thereafter.
(i) The number of moles of vessel 1 equals the number of moles of in vessel 2.
(ii) The electrical conductivity of (in water) is higher than that of (in water).
(iii) The number of moles of ions in (water) is greater than the number of moles of ions in (water).
Which of the statements out of are correct?